Wetenschap
Hoe eiwitten samenkomen, speelt mogelijk een ondergewaardeerde rol bij ziekte
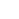
Grafisch abstract. Krediet:Ontwikkelingscel (2022). DOI:10.1016/j.devcel.2022.06.010
Dankzij de vooruitgang in de genomica in de afgelopen decennia, kennen onderzoekers nu de genetische mutaties die verantwoordelijk zijn voor veel ziekten. Onderzoekers weten echter vaak nog steeds niet hoe de mutatie tot de ziekte leidt - wat het in cellen verandert om symptomen te veroorzaken. Het uitzoeken van dit ontbrekende stukje, het ziektemechanisme, bevordert niet alleen het begrip van de ziekte, maar kan ook essentieel zijn voor het ontwikkelen van een behandeling of preventie. Als onderzoekers bijvoorbeeld weten dat een mutatie leidt tot de aanmaak van een defect eiwit, en dat de opbouw van dit defecte eiwit de ziekte veroorzaakt, dan kunnen ze medicijnen ontwerpen die zullen leiden tot de vernietiging van het eiwit.
Onderzoekers in het laboratorium van Whitehead Institute-lid Richard Young hebben een proces bestudeerd dat biomoleculaire condensatie wordt genoemd en dat helpt te organiseren waar moleculen in cellen terechtkomen, en ze vermoedden dat verstoring van dit proces een ondergewaardeerd, algemeen ziektemechanisme zou kunnen zijn. Condensaten zijn structuren die ontstaan wanneer veel moleculen losjes in elkaar grijpen om een druppel te creëren die zich afscheidt van de andere inhoud van de cel, zoals een oliedruppel die in water is gesuspendeerd. Het nieuwste onderzoek van het Young lab, gepubliceerd in Developmental Cell op 8 juli, suggereert dat verstoring van condensaten inderdaad alomtegenwoordig is in alle ziektetypes door het hele lichaam. De paper biedt onderzoekers een catalogus van waarschijnlijke gevallen. Ze hopen dat deze catalogus zal worden gebruikt om meer inzicht te krijgen in ziekten waarbij condensaten een rol spelen en om uiteindelijk nieuwe therapieën voor die ziekten te ontwikkelen.
Groeiend bewustzijn van condensaatbiologie
Alleen bepaalde moleculen - sommige eiwitten en RNA's - vormen condensaten, omdat ze regio's bevatten die losjes aan elkaar kunnen binden. De meeste eiwitten hebben regio's die alleen zeer sterk binden aan specifieke moleculen, zoals een sleutel die stevig in een slot steekt. Condensaatvormende eiwitten hebben in plaats daarvan de neiging om veel losse, minder specifieke verbindingen met elkaar te maken, die in hoge concentraties in elkaar grijpen totdat ze een druppel vormen. Cellen gebruiken condensaten om moleculen te verzamelen waar ze nodig zijn in de cel - moleculen die bijvoorbeeld nodig zijn om genen te transcriberen, kunnen condensaten vormen in de buurt van die genen.
Condensaatonderzoekers, waaronder Young, die ook hoogleraar biologie is aan het Massachusetts Institute of Technology, hebben de afgelopen jaren aangetoond dat condensaten een rol spelen in veel belangrijke cellulaire processen. Condensate disruption has also been shown to occur in a small but growing number of diseases, in particular neurodegenerative disorders. However, when researchers are hunting for disease mechanisms, condensate disruption is not often considered. Given how commonly condensates play a role in healthy biology, Young lab researchers suspected that their disruption might be common in disease. Postdoc in the Young lab and co-first author Salman Banani, who is also a pathologist at the Brigham and Women's Hospital, began suspecting as much during his clinical training, while looking at genetic sequencing data for cancer patients.
"The goal was to assess the clinical relevance of the mutations in the tumors' genomes and how that might impact care for each patient. I noticed that many of the mutations I was examining were suspiciously affecting regions of the protein that I thought might be involved in condensation," Banani says. "That led me to wonder how often human disease mutations affect condensate forming regions, if we had overlooked a potentially widespread cause of human disease, and whether pathologists should consider condensation properties in any of our assessments of mutations."
When Banani joined the Young lab, the potential clinical significance of mutations in condensate-forming proteins was fresh on his mind. Graduate students in the Young lab and co-first authors on the paper Lena Afeyan and Susana Wilson Hawken, who had been studying how drugs interact with condensates, were also eager to tackle the question of how broadly condensates contribute to disease.
Condensates and disease
The researchers compiled a list of proteins thought to form condensates, and mapped the locations of condensate-forming regions within each protein. They also made a list of genetic mutations known to cause or contribute to a wide variety of diseases, including diseases in which a mutation to a single gene is responsible for the disease and a number of cancers. Next, the researchers mapped each mutation to the part of each protein it affects. From this work, they created a list of disease-causing mutations that occur within the condensate-forming regions of the proteins. They hypothesized that these mutations would be most likely to affect the proteins' ability to form condensates.
The researchers ended up with a catalog of more than 36,000 disease-causing mutations, affecting more than 1,000 proteins, that likely disrupt condensates. The mutations in the catalog contribute to more that 1,700 diseases, including more than 550 cancers, collectively affecting every part of the body. Now, the researchers hope, people studying those diseases can use their catalog as a jumping off point to test if condensate disruption may in fact be a mechanism underlying the disease. This may in turn provide opportunities to develop therapies that target condensates.
"If we now know that a mutation affects a protein that likely resides in a condensate, we can test in cells to see whether and how the mutation affects the condensates and whether this effect is relevant for the underlying disease," Wilson Hawken says. "Then we could test a panel of drugs to see if we could rescue normal condensate formation and if this would be a good way to treat that particular disease."
The researchers tested a sample of the proteins and mutations from their catalog to verify that the catalog is a good predictor of mutations that disrupt condensates. They selected thirteen proteins that are able to form condensates in mouse embryonic stem cells, and introduced relevant disease-causing mutations to the proteins within those cells. Of the fifteen mutations they introduced, thirteen disrupted condensates; this high rate of disruption suggests that the catalog is a strong predictive tool.
Interestingly, different mutations disrupted condensates in different ways. The most common effect was to reduce the proteins' ability to join condensates. However, one mutation instead increased the proteins' ability to join condensates, and another affected where the condensates ended up in cells—all over the cell instead of contained within the nucleus. Determining the specific way in which a mutation affects condensates will be important for understanding disease mechanisms and developing drugs to reverse the mutation's effects.
"This catalog is a great starting point to ask lots of questions about condensate dysregulation as a disease mechanism, such as how do changes in the properties of condensates affect the cellular processes happening in these condensates? We and others believe condensates could have profound implications for disease and drug development, just as they have had in basic molecular biology, and our hope with this catalog is that we can lower the barrier to entry for many more disease researchers to start studying them," Afeyan says.
The researchers hope that their catalog proves useful in others' research, and they also hope that it raises awareness of the likely pervasiveness of condensate dysregulation in diseases, even beyond the instances in the catalog.
"There are likely many cases not covered by the catalog, such as when the mutation does not directly affect the condensate-forming protein, but rather affects one of its regulators," Young says. "I think we are just seeing the tip of the iceberg in terms of the prevalence of condensate dysregulation in disease. My vision for the future is that researchers will consider a condensate model among the conventional models when searching for the potential underlying disease mechanism of any mutation."
 Hoe heet moet water zijn om kunststof te smelten?
Hoe heet moet water zijn om kunststof te smelten?  Onderzoekers ontwikkelen micro-elektro-fluïdische sonde (MeFP) om cellen te isoleren en te modelleren
Onderzoekers ontwikkelen micro-elektro-fluïdische sonde (MeFP) om cellen te isoleren en te modelleren Vloeibaar platina bij kamertemperatuur:de koele katalysator voor een duurzame revolutie in de industriële chemie
Vloeibaar platina bij kamertemperatuur:de koele katalysator voor een duurzame revolutie in de industriële chemie Welke klassen moet je volgen op de middelbare school als je een chemisch ingenieur wilt worden?
Welke klassen moet je volgen op de middelbare school als je een chemisch ingenieur wilt worden?  Metalen organische raamwerken gemaakt om als vloeistof te fungeren
Metalen organische raamwerken gemaakt om als vloeistof te fungeren
 De verbinding tussen water en menselijke biologie is nu belangrijker dan ooit
De verbinding tussen water en menselijke biologie is nu belangrijker dan ooit Toegankelijke gezondheidszorg kan de sleutel zijn tot het oplossen van de klimaatcrisis
Toegankelijke gezondheidszorg kan de sleutel zijn tot het oplossen van de klimaatcrisis Vervuiling in de Aziatische tropopauze-laag is afkomstig van menselijke activiteiten en natuurlijke bronnen, studie vondsten
Vervuiling in de Aziatische tropopauze-laag is afkomstig van menselijke activiteiten en natuurlijke bronnen, studie vondsten Satellieten laten gletsjers van de wereld zien die sneller dan ooit smelten
Satellieten laten gletsjers van de wereld zien die sneller dan ooit smelten Klimaatverandering:hoe kan kunstmatige fotosynthese bijdragen aan het beperken van de opwarming van de aarde?
Klimaatverandering:hoe kan kunstmatige fotosynthese bijdragen aan het beperken van de opwarming van de aarde?
Hoofdlijnen
- Deskundigen maken zich zorgen over rauw vlees-diëten voor katten en honden
- Vergelijking voor glucosemetabolisme
- De sleutelrol van enzymen in de embryonale ontwikkeling begrijpen
- Soorten instrumenten die worden gebruikt voor het meten van lichaamstemperaturen
- Retrovirus versus DNA-virus
- 10 fascinerende feiten over de nieuwe levensboom-evolutiekaart
- Hoe een mRNA-sequentie
- Celstructuurdefinities
- Revolutionaire beeldvormingstechniek maakt gebruik van CRISPR om DNA-mutaties in kaart te brengen
- Type energie geproduceerd door fotosynthese

- Bloem trekt insecten aan door zich voor te doen als paddenstoel

- Het modelleren van antimicrobieel gebruik en resistentie in Canadese koppels kalkoenen
- Nieuwe ontdekking:gewone kwallen zijn eigenlijk twee soorten

- Wat zijn de effecten van isolatie in de geest?
 Een puntgrafiek maken
Een puntgrafiek maken  Biodiversiteit lijdt als het klimaat warmer wordt
Biodiversiteit lijdt als het klimaat warmer wordt Gebakken meteorieten geven aanwijzingen voor planetaire atmosferen
Gebakken meteorieten geven aanwijzingen voor planetaire atmosferen Nabij-infraroodsonde decodeert de dynamiek van telomeren
Nabij-infraroodsonde decodeert de dynamiek van telomeren Zwerfvuilprobleem aan de beschermde kusten van Engeland
Zwerfvuilprobleem aan de beschermde kusten van Engeland Wat is de fysieke expressie van een allel?
Wat is de fysieke expressie van een allel?  Waarom volhouden dat je niet racistisch bent, kan averechts werken?
Waarom volhouden dat je niet racistisch bent, kan averechts werken? Neanderthaler-achtige kenmerken in 450, 000 jaar oude fossiele tanden van het Italiaanse schiereiland
Neanderthaler-achtige kenmerken in 450, 000 jaar oude fossiele tanden van het Italiaanse schiereiland
- Elektronica
- Biologie
- Zonsverduistering
- Wiskunde
- French | Italian | Spanish | Portuguese | Swedish | German | Dutch | Danish | Norway |

-
Wetenschap © https://nl.scienceaq.com