Wetenschap
Diamanten onthullen neurale geheimen
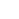
Een prototype van een diamant-voltage imaging-microscoop gebouwd door natuurkundigen aan de Universiteit van Melbourne. Een kleine elektrode wordt boven de diamantchip opgehangen om de prestaties van het apparaat te testen. Een groene laser die van onderaf schijnt, zorgt voor fluorescentie-excitatie op de chip. Krediet:auteur verstrekt, Universiteit van Melbourne
De hersenen zijn misschien wel een van de meest complexe structuren in het bekende universum.
Voortdurende vooruitgang in ons begrip van de hersenen en ons vermogen om een groot aantal neurologische ziekten effectief te behandelen, is afhankelijk van het steeds gedetailleerder onderzoeken van de neurale microcircuits van de hersenen.
Een klasse van methoden voor het bestuderen van neurale circuits wordt spanningsbeeldvorming genoemd. Met deze technieken kunnen we de spanning zien die wordt gegenereerd door de afvurende neuronen van onze hersenen en ons vertellen hoe netwerken van neuronen zich in de loop van de tijd ontwikkelen, functioneren en veranderen.
Tegenwoordig wordt spanningsbeeldvorming van gekweekte neuronen uitgevoerd met behulp van dichte arrays van elektroden waarop cellen worden gekweekt (of gekweekt), of door lichtemitterende kleurstoffen toe te passen die optisch reageren op veranderingen in spanning op het oppervlak van de cel.
Maar het detailniveau dat we met deze technieken kunnen zien, is beperkt.
De kleinste elektroden kunnen individuele neuronen, zo'n 20 miljoenste van een meter breed, niet op betrouwbare wijze onderscheiden, om nog maar te zwijgen van het dichte netwerk van verbindingen op nanoschaal dat zich daartussen vormt, en er is al meer dan twee decennia geen significante technologische vooruitgang geboekt op dit gebied.
Bovendien heeft elke elektrode zijn eigen bedrade verbinding en versterker nodig, wat aanzienlijke beperkingen oplegt aan het aantal elektroden dat tegelijkertijd kan worden gemeten.
Kleurstoffen kunnen deze beperkingen overwinnen door de spanning draadloos als licht af te beelden - dit betekent dat de complexe elektronica uit de buurt van de cellen in een camera kan worden geplaatst.
Het resultaat is een hoge resolutie over grote gebieden, in staat om elk individueel neuron in een groot netwerk te onderscheiden. But there are limitations here too, the voltage responses of state-of-the-art dyes are slow and unstable.
Our recent research published in Nature Photonics , explores a new type of a high speed, high resolution and scalable voltage imaging platform created with the aim of overcoming these limitations—a diamond voltage imaging microscope.
Developed by a team of physicists from the University of Melbourne and RMIT University, the device uses a diamond-based sensor that converts voltage signals at its surface directly into optical signals—this means we can see electrical activity as it happens.
The conversion uses the properties of an atom-scale defect in the diamond's crystal structure known as the nitrogen-vacancy (NV).
NV defects can be engineered by bombarding the diamond with a nitrogen ion beam using a special type of particle accelerator. The fabrication of the sensor begins with using this process to create a high-density, ultra-thin layer of NV defects close to the diamond's surface.
You can think of each NV defect as a bucket that holds up to two electrons. When this bucket is empty, the NV defect is dark. With one electron, the NV defect emits orange light when illuminated by a laser—this property is known as fluorescence. With two electrons, the color of the fluorescence becomes red.
A previously discovered property of NV defects is that the number of electrons they hold—and the resulting fluorescence—can be controlled with a voltage. Unlike dyes, the voltage response of an NV defect is very fast and stable.
Our research aims to overcome the challenge of making this effect sensitive enough to image neuronal activity.
On the diamond's surface, the crystal structure ends with a layer one atom thick, made up of hydrogen and oxygen atoms. The NV defects closest to the surface are the most sensitive to changes in voltage outside the diamond, but they are also highly sensitive to the atomic makeup of the surface layer.
Too much hydrogen and the NVs are so dark that the optical signals we are looking for cannot be seen. Too little hydrogen and the NVs are so bright that the small signals we are after are completely washed out.
So, there's a "Goldilocks' zone" for voltage imaging, where the surface has just the right amount of hydrogen.
To reach this zone, our team developed an electrochemical method for removing hydrogen in a controlled way. By doing this, we've managed to achieve voltage sensitivities two orders of magnitude better than what has been previously reported.
We tested our sensor in salty water using a microscopic wire 10-times thinner than a human hair. By applying a current, the wire can produce a small cloud of charge in the water above the diamond. The formation and subsequent diffusion of this charge cloud produces small voltages at the diamond surface.
By capturing these voltages through a high-speed recording of the NV fluorescence, we can determine the speed, sensitivity and resolution of our diamond imaging chip.
We were able to further boost sensitivity by patterning the diamond's surface into 'nanopillars'—conical structures with the NV centers embedded in their tips. These pillars funnel the light emitted by the NVs towards the camera, dramatically increasing the amount of signal we can collect.
With the development of the diamond voltage imaging microscope for detecting neuronal activity, the next step is the recording of activity from cultured neurons in vitro—these are experiments on cells grown outside their normal biological context, otherwise known as test-tube or petri-dish experiments.
What differentiates this technology from existing state-of-the-art in vitro techniques is the combination of high spatial resolution (on the order of a millionth of a meter or less), large spatial scale (a few millimeters in each direction—which for a network of neurons in mammals is quite vast), and complete stability over time.
No other existing system can simultaneously offer these three qualities, and it's this combination that will allow our made-in-Melbourne technology to make a valuable contribution to the work of neuroscientists and neuropharmacologists globally.
Our system will aid these researchers in pursuing both fundamental knowledge and the next generation of treatments for neurological and neurodegenerative diseases. + Explore further
New method enables long-lasting imaging of rapid brain activity in individual cells deep in the cortex
 Kristallografie biedt blauwdrukken voor gevechtsplannen voor het aanvallen van ziekteverwekkende bacteriën
Kristallografie biedt blauwdrukken voor gevechtsplannen voor het aanvallen van ziekteverwekkende bacteriën Wetenschapsprojecten over het scheiden van olie en water
Wetenschapsprojecten over het scheiden van olie en water Wat zijn Spectator-ionen?
Wat zijn Spectator-ionen?  Doorbraak bereikt bij het verbeteren van de ionische geleidbaarheid van brandstofcelmaterialen
Doorbraak bereikt bij het verbeteren van de ionische geleidbaarheid van brandstofcelmaterialen Het verminderen van vallen verhoogt de prestaties van organische fotodetectoren
Het verminderen van vallen verhoogt de prestaties van organische fotodetectoren
 China, Japan haalt brandbaar ijs uit de zeebodem
China, Japan haalt brandbaar ijs uit de zeebodem Diep gaan om de geheimen van de aardbevingen in Japan te leren
Diep gaan om de geheimen van de aardbevingen in Japan te leren Erosie van de Arctische kust onthuld door dronebeelden
Erosie van de Arctische kust onthuld door dronebeelden NASA analyseert waterdampconcentratie van tropische cycloon Herolds
NASA analyseert waterdampconcentratie van tropische cycloon Herolds Stedelijke hitte-eilanden hebben invloed op de temperatuur en gezondheid van het bladerdak, studie zegt:
Stedelijke hitte-eilanden hebben invloed op de temperatuur en gezondheid van het bladerdak, studie zegt:
Hoofdlijnen
- Vroegtijdige waarschuwing gezondheids- en welzijnssysteem kan boeren miljoenen ponden besparen
- Veranderingen in het bladerdak vergemakkelijkten de evolutie van het allereerste glijdende reptiel, suggereert nieuw onderzoek
- Wanneer één referentiegenoom niet genoeg is
- Nieuw fundamenteel inzicht in de strijd tegen bacteriën
- Bereken de percentages van adenine in een DNA-streng
- Walvisstranden:vijf vragen beantwoord
- Cheerleading Science Fair Projectideeën
- De 3 soorten bacteriën
- De isovormen van het HP1-eiwit reguleren de organisatie en structuur van heterochromatine
- Zelfassemblerende reagentia met instelbare kleuren en helderheid maken zeer multiplex tagging mogelijk, microscopische beeldvorming

- Onderzoekers onderzoeken hoe terahertz-golven interageren met lenzen met schot in de roos
- Aan de rand van chaos:nieuwe methode voor analyse van stabiliteit van exoplaneten
- Slim oppervlak maakt geavanceerde manipulatie van druppels mogelijk

- Bemanningen zorgen voor een knaller om het Deep Underground Neutrino-experiment naar de volgende fase te brengen

 Bedrijven die de pandemie hebben overleefd, zullen herstellen
Bedrijven die de pandemie hebben overleefd, zullen herstellen Sensoren op nanoschaal meten ongrijpbare waterstanden in bladeren
Sensoren op nanoschaal meten ongrijpbare waterstanden in bladeren NASA volgt zware regenval na de tropische cycloon Michaels naar het noordoosten van de VS
NASA volgt zware regenval na de tropische cycloon Michaels naar het noordoosten van de VS Kustaanpassing tegen zeespiegelstijging is economisch zinvol
Kustaanpassing tegen zeespiegelstijging is economisch zinvol Droogte verdroogt zuidelijk Afrika, miljoenen geconfronteerd met honger
Droogte verdroogt zuidelijk Afrika, miljoenen geconfronteerd met honger De voordelen van het bestuderen van cellen onder een lichtmicroscoop
De voordelen van het bestuderen van cellen onder een lichtmicroscoop Afbeelding:ESO-telescoop legt een spectaculaire kosmische dans vast
Afbeelding:ESO-telescoop legt een spectaculaire kosmische dans vast Nieuwe sonde voor stofwisselingsziekten
Nieuwe sonde voor stofwisselingsziekten
- Elektronica
- Biologie
- Zonsverduistering
- Wiskunde
- French | Italian | Spanish | Portuguese | Swedish | German | Dutch | Danish | Norway |

-
Wetenschap © https://nl.scienceaq.com