Wetenschap
Onderzoeker bespreekt de zelfassemblage van materialen om verschillende patronen op nanoschaal te maken

Materiaalwetenschapper Gregory Doerk in het materiaalverwerkingslab bij CFN. Krediet:Brookhaven National Laboratory
Sommige materialen hebben het unieke vermogen om zichzelf te assembleren tot georganiseerde moleculaire patronen en structuren. Materiaalwetenschapper Gregory Doerk van de Electronic Nanomaterials Group van het Center for Functional Nanomaterials (CFN) - een Office of Science User Facility van het Amerikaanse Department of Energy (DOE) in het Brookhaven National Laboratory - maakt gebruik van dit vermogen in materialen die blokcopolymeren worden genoemd. Met behulp van deze zelf-assemblerende materialen, die ketens hebben van twee of meer verschillende moleculen die met elkaar zijn verbonden door chemische bindingen, Doerk regisseert de vorming van dergelijke patronen en structuren op nanoschaal. Het uiteindelijke doel is om deze architecturen op nanoschaal te gebruiken om de eigenschappen van materialen te beheersen voor toepassingen zoals conversie en opslag van zonne-energie, katalyse, en optica.
Zelfassemblage heeft de laatste tijd veel aandacht gekregen als nanofabricagebenadering. Waarin verschilt het van de benadering die van oudsher wordt gebruikt?
In grote lijnen, er zijn twee platforms voor nanofabricage. Een platform is top-down nanofabricage, dat is de traditionele benadering die wordt gebruikt om computerchips en andere micro-elektronica te maken. Met licht worden patronen gemaakt die vervolgens in siliciumwafels worden gesneden. De patroontechniek waarbij gebruik wordt gemaakt van licht wordt optische lithografie genoemd. De andere benadering is bottom-up nanofabricage, of moleculaire zelfassemblage. De eigenschappen van de materialen zijn gecodeerd in hun zwakke interacties, die ervoor zorgen dat bepaalde materialen samenkomen en specifieke configuraties vormen - een beetje zoals Legoblokjes, maar de stenen bouwen allemaal vanzelf op om een structuur te vormen.
Lithografie is over het algemeen sneller - en produceert betrouwbaarder de ontworpen structuren - maar vereist dure, complexe hulpmiddelen. Zelfmontage is vaak langzamer en minder voorspellend, maar het is goedkoop en kan gemakkelijker zijn. Er zijn manieren om de twee benaderingen te combineren, en die combinatie staat bekend als gerichte zelfassemblage. Zelf-assemblerende materialen, zoals dunne films van blokcopolymeren, worden besteld met behulp van sjablonen die zijn gemodelleerd door standaardlithografie. Gerichte zelfassemblage verbetert de huidige productieprocessen, het spectrum van mogelijke patroongeometrieën uitbreiden, en verlaagt de kosten van nanofabricage.
Uw zelfassemblageonderzoek is gericht op blokcopolymeren. Wat maakt deze materialen zo bijzonder?
Sinds de jaren vijftig, mensen hebben triblokcopolymeren (drie polymeren samengevoegd) gebruikt in materialen zoals synthetisch rubber. De meeste polymeren zullen niet mengen. Proberen polymeren te mengen is net zoiets als proberen olie en water te mengen.
Maar in blokcopolymeren, de polymeerketens zijn chemisch aan elkaar gebonden. Bijvoorbeeld, diblok (twee) copolymeerketens zijn verbonden via een covalente binding. Deze binding frustreert hun drang om te 'ontmengen'. In plaats daarvan, als blokcopolymeren energie krijgen om hun ketens te mobiliseren, bijvoorbeeld door de polymeerfilm op een hete plaat te gloeien (verhitten) tot boven de glasovergangstemperatuur (wanneer een polymeer overgaat van een harde, glasachtig materiaal tot een zacht, rubberachtige) - de ketens zullen zich opnieuw configureren en samenvoegen tot fasegescheiden domeinen op nanoschaal. Deze domeinen zijn geordend in patronen op nanoschaal op basis van inherente eigenschappen van het blokcopolymeer. Bijvoorbeeld, laten we zeggen dat we twee polymeren hebben, A en B, samengevoegd (diblokcopolymeer). De lengte van blok A ten opzichte van de lengte van beide blokken, de totale lengte van de polymeerketens, en de eigenschappen van de polymeren en de sterkte van hun interactie (hoeveel de polymeren uit elkaar willen trekken) bepalen de grootte en morfologie, of vorm, van de resulterende structuren. Als de ketens te kort zijn of hun afstotende interacties te zwak zijn, de polymeren zullen mengen, waardoor de geordende staat een wanordelijke staat wordt en er zich dus geen patroon zal vormen.
Bij CFN, een groot deel van ons werk is het overbrengen van de patronen en structuren die door zelfassemblage zijn gemaakt, elektronenstraallithografie, of optische lithografie in onze Nanofabrication Facility in andere materialen om materiaaleigenschappen te controleren. Een goed voorbeeld hiervan is het werk van CFN-directeur Chuck Black waarbij blokcopolymeren worden gebruikt om een zelf-geassembleerd patroon te creëren dat dient als sjabloon voor het etsen van kegeltjes van nanoformaat op siliciumoppervlakken. Met deze nanotexturen de siliciumoppervlakken veranderden van reflecterende spiegels in volledig zwart. Dat is een manier waarop we blokcopolymeren kunnen gebruiken.
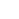
De lengte van de polymeerblokken (rode en blauwe golvende lijnen) en de sterkte van hun interactie bepalen de vorm van de resulterende patronen in zelfassemblage van blokcopolymeer:(van links naar rechts) bollen, cilinders, en lamellen (platen). Krediet:Gregory Doerk
Maar we willen het scala aan dingen dat we kunnen doen met zelfmontage uitbreiden - en dus het scala aan toepassingen. Het diversifiëren van de mogelijke patronen met zelfassemblage van blokcopolymeer is een groot deel van mijn onderzoek.
Heb je dit assortiment kunnen uitbreiden?
Een manier waarop ik geïnteresseerd ben in het uitbreiden van wat we kunnen doen door middel van zelfassemblage, is door anorganische replica's te maken van de zelfgeassembleerde structuren. We can accomplish this replication through a process called infiltration synthesis.
Bijvoorbeeld, say you have a block copolymer that forms lamellae (stripes) perpendicular to a substrate. Using an atomic-layer-deposition tool, it is possible to infiltrate those lamellae with a metal oxide and then remove the polymer, leaving behind metal oxide lines in a pattern determined by the polymer self-assembly. It is even possible to perform self-assembly and replica formation on top of previous replicas in an iterative way. What is really interesting is that the topography from the initial layer actually acts as a template dictating how polymer domains in the next layer line up. In the case of a second layer of lamellae perpendicular with the substrate, a self-assembled nanomesh is naturally created.
What other ways can you expand the range of self-assembled patterns?
One area in which I have collaborated with other members of the Electronic Nanomaterials Group is to study the blending of block copolymers that form lamellae with block copolymers that form cylinders. The really cool thing we learned is that you can precisely control which of these two patterns emerge in different areas of a substrate through an approach we call selective directed self-assembly. In a diblock copolymer, one of the blocks may be relatively more hydrophobic (water repelling) and the other may be relatively more hydrophilic (water attracting). So if a chemical pattern was made up of alternating hydrophobic and hydrophilic lines, the block copolymer molecules would self-assemble accordingly. By changing the spacing and width of the chemical line gratings (patterns) on the substrate, we were able to direct the self-assembling blocks into specific arrangements—either forming striped patterns (lamellae) or hexagonal dot arrays (cylinders). We can locally adjust the spacing and linewidth of the underlying chemical template to precisely control exactly where these line or dot patterns form on the same substrate, te.
How big are the features of these self-assembled nanoscale patterns?
Block copolymers typically self-assemble into ordered periodic structures with a tunable repeat spacing between around 25 to 50 nanometers. Werkelijk, one of the projects I am currently working on is to increase the size range over which block copolymers form patterns. There is a lot of work in the scientific community to go to smaller and smaller sizes (approaching a few nanometers) for lithographic applications such as making computer chips. But for certain applications, you need larger sizes.
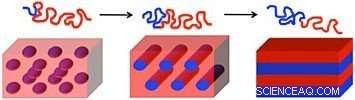
Plan view (top) and cross-sectional (bottom) scanning electron microscope images of an inorganic nanomesh created through iterative self-assembly and infiltration synthesis. Credit:Gregory Doerk
Bijvoorbeeld, the wavelength range of visible light is about 400 (purple) to 700 (red) nanometers. Even 400 nanometers is still about 10 times larger than the approximately 40-nanometer length scale possible with most block copolymers. Als resultaat, light does not "see" the individual features of block copolymers.
Echter, patterns with features closer to 200 nanometers in size can influence light in new ways, and I am working to scale block copolymer assembly to these sizes. One exciting application is using this approach to make "structural colors." Colors are typically made using dyes or pigments. Echter, structural colors emerge from the way the light interacts with the nanomaterial—and so potentially one could make new types of lower-power displays through structural color.
Helaas, the process of forming patterns from block copolymers slows drastically, and even stops altogether, for these larger sizes. The development of self-assembled patterns is impeded by defective structures that form at the start of self-assembly. For these defects to "heal, " the block polymer must reconfigure, which involves pulling one chain through the domain of the other polymer, overcoming a large energy barrier to do so. As the size of the polymers increases, the energy barrier goes up exponentially—so exponentially slower healing!
Have you come up with any solutions to overcome this challenge?
My colleagues and I found that blending small-molecular-weight homopolymers (polymers made up of the same type of molecules) with the block copolymers makes it easier for the block copolymer chains to move around. Adding homopolymers promotes dramatic increases in the size of well-ordered pattern areas, or "grains." Echter, this addition alone does not let us form patterns with larger-size features from block copolymers. We also need to anneal the materials in a solvent vapor. Solvent vapor annealing involves putting a volatile solvent in the region of the diblock copolymer, causing the polymer film to swell. As the polymer film swells, the solvent molecules intersperse between polymer chains. This process has a plasticizing effect, making it easier for the polymer to move. So both mixing the block copolymer with a homopolymer and swelling it with a solvent are needed to speed the formation of large-scale patterns.
After annealing, we image the resulting patterns with scanning electron microscopes at CFN. We also perform x-ray scattering experiments at the Complex Materials Scattering beamline at Brookhaven Lab's National Synchrotron Light Source II [also a DOE Office of Science User Facility] to get a measure of the periodicity during the annealing process so we can determine how quickly the material self-assembles into an ordered pattern.
How do all of the different self-assembled patterns you generate translate to possible applications?
The work we do at CFN establishes the basis for producing new materials. Bijvoorbeeld, consider the mesh pattern I described. My colleague Chang-Yong Nam and I are working on making a mesh of zinc oxide nanowires through infiltration synthesis of block copolymers. Zinc oxide is a versatile semiconducting material, and these nanowires have a lot of surface area, making them attractive for a number of applications—including photoelectrochemical water splitting (a way of converting sunlight into fuel by splitting water into hydrogen and oxygen). These semiconducting nanowires are also very responsive to environmental cues like light or chemicals. Given their large area uniformity, the meshes could be easily integrated into widely deployable gas sensors.
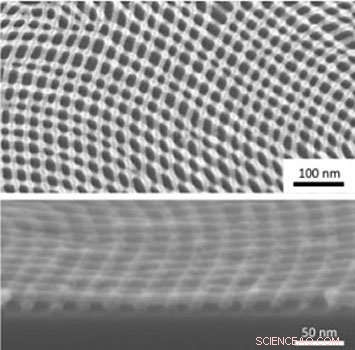
Without blending, the size of grains (single-color regions) of a diblock copolymer with a molecular weight of 36 kilograms/mole barely changes with thermal annealing over time (top row). Blending the block copolymer with homopolymers increases the grain size and speeds up the ordering process (bottom row). Credit:Gregory Doerk
How did you come to join CFN?
After graduate school, I completed a postdoctoral appointment working at IBM in California. It was there that I learned about block copolymers, using them to make patterns for microelectronics. I did that for three years, and then in 2013 I joined a research group at HGST, a subsidiary of Western Digital that sells hard disk and solid-state drives. Aanvankelijk, I worked on a project to create patterned nanoscale magnetic media onto which data is written to and read from, in order to enhance data density and stability.
Following that project, I moved into another area that was not very research oriented. At about this time, the current CFN Director, Chuck Black, gave a talk at HGST. Chuck's talk piqued my interest in CFN, and soon thereafter a position that very well fit my skills opened up. I applied, and joined CFN in 2015.
What was it like coming from industry to a national lab setting?
It definitely takes some getting used to. There is a lot less pressure in some ways but more pressure in other ways. At CFN, you are expected to develop a lot more on your own as far as what direction you need to pursue on the basis of what is valuable to the lab. In industrie, the goals of the project are clear, and there are deliverables to keep you on the narrow path to achieving those goals. This is not to say that I did not do exploratory research for a portion of my time in industry.
The other difference at CFN is that I am more involved with the academic and industrial community at large. I have to manage my time so that I can perform my own research and help users with their research. I find it really cool that researchers from all over the world come to CFN. The talent and expertise of the staff are what make the CFN so great. The staff know backwards and forwards what they are doing in the areas they specialize in, and they are dedicated to helping others.
What are some of the ways you have helped users?
I regularly show users how to do block copolymer self-assembly and the etching process to transfer patterns into a substrate. Some users have employed these patterns as superhydrophobic textures to manipulate the flow of liquids in microfluidic devices, bijvoorbeeld. Vaak, I help users develop a process when they need self-assembled patterns of varying sizes or shapes, to be applied to different substrates.
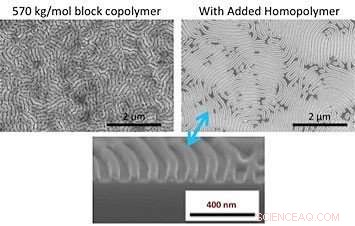
A comparison of scanning electron microscope images after solvent vapor annealing of a large block copolymer (top left) and the same copolymer with added homopolymer (top right) shows that the homopolymer can significantly improve pattern quality. The bottom image is a cross-sectional image of the top right sample. Credit:Gregory Doerk
I also help with the lithographic patterning of unusual materials. Bijvoorbeeld, one user I am working with is trying to pattern protein hydrogels, looking at how they mechanically respond to swelling and de-swelling to understand how they might operate if injected into the body. Such protein hydrogels could have applications in biosensing, medicijnafgifte, and wound healing.
Some users are interested in solvent vapor annealing, which is a very tricky process to control. So I am building a system with feedback control that can be set to maintain a solvent fraction of any given solvent. Otherwise, if the amount of solvent varies over time, the self-assembly might not work at all (too little solvent) or result in a disordered state (too much solvent).
How did you become interested in research?
As an undergraduate at Case Western Reserve University, I majored in chemical engineering. One of my summer internships involved doing fuel cell work at a research lab. This internship led to another one at a fuel cell start-up, where I worked on sensors and solar cells. These internships gave me the opportunity to come up with ideas of my own and try different things. This exploration spurred me to pursue a doctoral degree at the University of California, Berkeley, where I continued my studies in chemical engineering.
I also think my undergraduate studies in philosophy, which I minored in, gave me a new understanding of the way science works. I really enjoyed reading about the philosophy of science, including books like Kuhn's The Structure of Scientific Revolutions. I learned how the views we often have are naïve or not accurate. There are lots of things people get wrong for a long time. But that does not mean they were not learning. Science is not perfect—but that is okay.
I was not the kid who grew up knowing he wanted to be a scientist. I liked philosophy and delving into critical thought. But I began to realize there is a lot of commonality between philosophy and science—in both fields, the aim is to gain knowledge about the world around us. But science is better because you can actually test things!
 Hoe bereken ik de hoeveelheid zuur om de pH van het water te verlagen?
Hoe bereken ik de hoeveelheid zuur om de pH van het water te verlagen?  Domino-achtige kristallisatie van glas
Domino-achtige kristallisatie van glas Klein maar imposant:titanium verandert het gedrag van gastheerroosteratomen
Klein maar imposant:titanium verandert het gedrag van gastheerroosteratomen Nieuwe oplosmiddelen om plantaardige cellulose af te breken voor bio-ethanol
Nieuwe oplosmiddelen om plantaardige cellulose af te breken voor bio-ethanol Stimulatie van kleine gebieden op celoppervlakken met vrije radicalen met behulp van een microfluïdische sonde
Stimulatie van kleine gebieden op celoppervlakken met vrije radicalen met behulp van een microfluïdische sonde
Hoofdlijnen
- Kikker en menselijke bloedcellen vergelijken en identificeren
- Onderzoekers ontdekken hoe cellen decennia later infecties onthouden
- Roofzuchtige bacteriën gevonden in studie van longmicrobioom van patiënten met cystische fibrose
- Bedwantsen proberen in je vuile was te komen
- Chimpstudie onthult hoe de hersenstructuur onze evolutie heeft gevormd
- Verschillende Agar-platen
- Voorbeelden van een recessief allel
- Overeenkomsten & verschillen tussen osmose en diffusie
- Inspanningen van de marine om walvissen te beschermen hebben beperkt effect
- Een nieuwe strategie voor het fabriceren van pn-overgangen in enkelkristallijne Si-nanodraden, draaien

- Op koolstof gebaseerde nanomaterialen laten veelbelovende resultaten zien tegen SARS-CoV-2 en 12 andere virussen

- nanoschaal, mega bereik
- Een moleculaire lichtschakelaar?... Gewoon water toevoegen
- 2-D gelaagde apparaten kunnen zichzelf met precisie in elkaar zetten

 Relatie vastgesteld tussen helderheid en dieet van zwarte gaten
Relatie vastgesteld tussen helderheid en dieet van zwarte gaten Hoe weet je dat een getal Rational
Hoe weet je dat een getal Rational Moderne melkboer:bedrijf biedt bezorging en ophaling van afvalvrije huishoudelijke benodigdheden en boodschappen
Moderne melkboer:bedrijf biedt bezorging en ophaling van afvalvrije huishoudelijke benodigdheden en boodschappen Een wereldrecord in het detecteren van extreem lage niveaus van gasonzuiverheden
Een wereldrecord in het detecteren van extreem lage niveaus van gasonzuiverheden Hoe maken we waterstof uit steenkool, en is het echt een schone brandstof?
Hoe maken we waterstof uit steenkool, en is het echt een schone brandstof? Het testen van de wateren van de Oost-Siberische Noordelijke IJszee suggereert dat de oorsprong van het verhoogde methaan een reservoir is in de Laptev-zee
Het testen van de wateren van de Oost-Siberische Noordelijke IJszee suggereert dat de oorsprong van het verhoogde methaan een reservoir is in de Laptev-zee Vloeibare honing, harige spinazie en glanzende appels:verrassende feiten over je eten
Vloeibare honing, harige spinazie en glanzende appels:verrassende feiten over je eten Seleniumankers kunnen de duurzaamheid van platinabrandstofcelkatalysatoren verbeteren
Seleniumankers kunnen de duurzaamheid van platinabrandstofcelkatalysatoren verbeteren
- Elektronica
- Biologie
- Zonsverduistering
- Wiskunde
- French | Italian | Spanish | Portuguese | Swedish | German | Dutch | Danish | Norway |

-
Wetenschap © https://nl.scienceaq.com