Wetenschap
Wat is hydroxide -ionenconcentratie als pH 8?
Hier leest u hoe u de hydroxide-ionenconcentratie (OH-) kunt berekenen als de pH 8 is:
1. Relatie tussen pH en POH
* pH + poh =14
2. Bereken poh
* poh =14 - ph
* poh =14 - 8
* poh =6
3. Bereken [oh-]
* [Oh-] =10^-poh
* [Oh-] =10^-6
* [Oh-] =1 x 10^-6 m
Daarom is de hydroxide-ionenconcentratie ([oh-]) 1 x 10^-6 m wanneer de pH 8 is.
 Hoe de kwaliteit van gecultiveerd land in China op verschillende manieren kan worden verbeterd
Hoe de kwaliteit van gecultiveerd land in China op verschillende manieren kan worden verbeterd  Waarom bestaan er zoveel klimaten in Noord- en Zuid -Amerika?
Waarom bestaan er zoveel klimaten in Noord- en Zuid -Amerika?  GPS zorgt voor nauwkeurigere neerslagvoorspellingen
GPS zorgt voor nauwkeurigere neerslagvoorspellingen Voorbeelden van planten die in water leven
Voorbeelden van planten die in water leven  Onderzoek zal landbeheerders helpen bij het nemen van een risicoanalyse-aanpak voor de nieuwe natuurbrandrealiteit
Onderzoek zal landbeheerders helpen bij het nemen van een risicoanalyse-aanpak voor de nieuwe natuurbrandrealiteit
Hoofdlijnen
- Een fysieke kaart van een genoom geeft familieledenposities genen met afstanden gemeten?
- Wat is de wetenschappelijke betekenis van diafragma?
- Vindt natuurlijke selectie plaats in tegenstelling tot evolutie?
- Wat tanden onthullen over de levens van moderne mensen
- Wat heb ATP om suiker te produceren?
- Welke kernen zou het minst stabiel zijn?
- Waarom is het proces van het maken van voedsel in een plant genaamd fotosynthese?
- De mitochondria is de site van?
- Wetenschappers passen CRISPR aan om diabetes epigenetisch te behandelen, nierziekte, spierdystrofie
- Biochemicus ontdekt veelbelovend enzym om kankercellen te bestrijden
- Dit rode lampje betekent:ga voor medische ontdekkingen

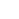
- In-situ inzichten op nanoschaal in de evolutie van vaste elektrolyt-interfaseschillen

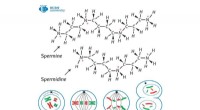
- Antibioticaresistentie bestrijden met fagen
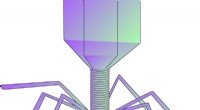
- Enigma van vetzuurmetabolisme opgelost - enzymvorm regelt activiteit
 Hashtags zijn misschien geen woorden, grammaticaal gesproken, maar ze helpen een boodschap te verspreiden
Hashtags zijn misschien geen woorden, grammaticaal gesproken, maar ze helpen een boodschap te verspreiden Welke verbindingen vinden octetuitbreiding op?
Welke verbindingen vinden octetuitbreiding op?  Herschik elke algebraïsche vergelijking met één eenvoudige regel
Herschik elke algebraïsche vergelijking met één eenvoudige regel Team publiceert onderzoek naar ongewone genevolutie in bacteriën
Team publiceert onderzoek naar ongewone genevolutie in bacteriën Wat is snelheid?
Wat is snelheid?  Natuurkundigen kunnen de sprongen van de kat van Schrodinger voorspellen (en hem uiteindelijk redden)
Natuurkundigen kunnen de sprongen van de kat van Schrodinger voorspellen (en hem uiteindelijk redden) Hoe nepnieuws in onze gedachten terechtkomt en wat u kunt doen om er weerstand aan te bieden
Hoe nepnieuws in onze gedachten terechtkomt en wat u kunt doen om er weerstand aan te bieden  Hoe ver zou je moeten bewegen voordat de sterrenbeelden van vorm lijken lijken?
Hoe ver zou je moeten bewegen voordat de sterrenbeelden van vorm lijken lijken?
- Elektronica
- Biologie
- Zonsverduistering
- Wiskunde
- French | Italian | Spanish | Portuguese | Swedish | German | Dutch | Danish | Norway |

-
Wetenschap © https://nl.scienceaq.com