Wetenschap
How do you react silver nitrate and iron?
Dit is wat er gebeurt:
* reactie: Fe(s) + 2AgNO₃(aq) → 2Ag(s) + Fe(NO₃)₂(aq)
* Verklaring:
* Iron (Fe) is more reactive than silver (Ag) according to the reactivity series of metals.
* Iron atoms will donate electrons to silver ions (Ag⁺) in the silver nitrate solution.
* This results in the formation of iron(II) nitrate (Fe(NO₃)₂) and solid silver (Ag) is deposited as a coating on the iron.
* Observaties:
* You will observe a grayish-white coating forming on the iron surface. This is the solid silver being deposited.
* The solution will turn pale green due to the formation of iron(II) nitrate.
Veiligheidsmaatregelen:
* Silver nitrate is corrosive and can cause skin irritation. Wear appropriate safety goggles and gloves when handling it.
* Iron(II) nitrate is also harmful. Avoid contact with skin and eyes.
* Voer het experiment uit in een goed geventileerd gebied.
Samenvattend: De reactie tussen zilvernitraat en ijzer is een klassiek voorbeeld van een enkele verplaatsingsreactie, wat resulteert in de vorming van zilvermetaal en ijzer (II) nitraat.
 Is waterdamp een mengsel of een zuivere stof?
Is waterdamp een mengsel of een zuivere stof?  Smelten de poolijskappen sneller dan we dachten?
Smelten de poolijskappen sneller dan we dachten?  Wat zijn de verschillende materialen die worden gebruikt om bevestigingsmiddelen te vervaardigen?
Wat zijn de verschillende materialen die worden gebruikt om bevestigingsmiddelen te vervaardigen?  Milieuvriendelijke katalysator voor het omzetten van methaan in bruikbare gassen met behulp van licht in plaats van warmte
Milieuvriendelijke katalysator voor het omzetten van methaan in bruikbare gassen met behulp van licht in plaats van warmte Waarom hebben vaste ionische verbindingen hoge smeltpunten?
Waarom hebben vaste ionische verbindingen hoge smeltpunten?
 Vrede, geen oorlog, verantwoordelijk voor ontbossing in gewapende conflictgebieden
Vrede, geen oorlog, verantwoordelijk voor ontbossing in gewapende conflictgebieden Wat is de studie van fysieke kenmerken van de werelden?
Wat is de studie van fysieke kenmerken van de werelden?  Onderzoekers tonen aan dat een verhoogd risico op roofdieren een adaptieve respons bij vogels kan oproepen
Onderzoekers tonen aan dat een verhoogd risico op roofdieren een adaptieve respons bij vogels kan oproepen  Internet van elektriciteit en koolstofvrije moleculen zal Europa helpen koolstofarmer te maken – rapport
Internet van elektriciteit en koolstofvrije moleculen zal Europa helpen koolstofarmer te maken – rapport Hoe helpt de milieuwetenschap ons de natuurlijke wereld te begrijpen?
Hoe helpt de milieuwetenschap ons de natuurlijke wereld te begrijpen?
Hoofdlijnen
- Zal het wegglijden of vastgrijpen:Wetenschappers vragen zich af:'Wat is slakkenslijm?'
- Het ontdekken van de geheimen van hoe chromosomen assembleren
- Wat zijn zoetwatermicroscopische organismen?
- Wat zijn de verschillende takken in de wetenschap?
- De wetenschap boekt vooruitgang op het gebied van veroudering. Maar willen we eeuwig leven?
- Kunnen we Ingenuity vervangen door een zwerm robotbijen?
- Wat zijn enkele pakkende wetenschapsprojecttitels voor het immuunsysteem?
- Wat is een monatomische anionennaam voor F?
- Wat zijn de reproductieve bloemenorganen?
- Russische wetenschappers ontwikkelden een sensor voor het detecteren van giftige stoffen in waterlichamen

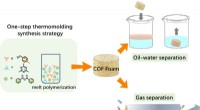
- Gebruik van smeltpolymerisatie om robuust covalent organisch raamwerkschuim te fabriceren
- Wittere tanden, zonder de verbranding

- Nieuwe enzymvrije strategie om organofosforresiduen van pesticiden te detecteren

- Wetenschappers ontwikkelen indoor-actieve fotokatalysator voor antivirale coating tegen verschillende COVID-varianten

 Nieuwe, door licht aangedreven katalysatoren kunnen helpen bij de productie
Nieuwe, door licht aangedreven katalysatoren kunnen helpen bij de productie Hoe lang zullen de Amerikaanse oliereserves meegaan?
Hoe lang zullen de Amerikaanse oliereserves meegaan?  Wat zei Aristarchus over het universum?
Wat zei Aristarchus over het universum?  Een nieuwe formule voor het creëren van chemische reacties - met koolhydraten
Een nieuwe formule voor het creëren van chemische reacties - met koolhydraten Gladde vloeistofoppervlakken verwarren mosselen
Gladde vloeistofoppervlakken verwarren mosselen wat gebeurt er als sedimenten zich in de loop van de tijd opbouwen?
wat gebeurt er als sedimenten zich in de loop van de tijd opbouwen?  Kepler-telescoop wenst goedenacht met laatste commando's
Kepler-telescoop wenst goedenacht met laatste commando's Energiestoot van het meest verre bekende sterrenstelsel zou een satelliet kunnen zijn die in een baan om de aarde draait
Energiestoot van het meest verre bekende sterrenstelsel zou een satelliet kunnen zijn die in een baan om de aarde draait
- Elektronica
- Biologie
- Zonsverduistering
- Wiskunde
- French | Italian | Spanish | Portuguese | Swedish | German | Dutch | Danish | Norway |

-
Wetenschap © https://nl.scienceaq.com