Wetenschap
What unit measures the mass of a single atom?
The unit that measures the mass of a single atom is the atomic mass unit (amu) .
Here's a bit more about it:
* Definitie: One atomic mass unit (amu) is defined as 1/12th the mass of a carbon-12 atom.
* Convenient: It's a very small unit, making it easier to express the masses of tiny atoms.
* Not the same as grams: It's important to remember that amu is not the same as grams.
* Conversie: 1 amu is approximately equal to 1.66053906660 × 10^-27 kg.
Laat het me weten als je nog andere vragen hebt!
 Een verbinding is wat of ionisch van aard?
Een verbinding is wat of ionisch van aard?  Wat is een voorbeeld van een vloeibaar-gasoplossing, en welke gas-gasoplossing?
Wat is een voorbeeld van een vloeibaar-gasoplossing, en welke gas-gasoplossing?  Acid Base Titration Sources of Error Improvements
Acid Base Titration Sources of Error Improvements  Wetenschappers gebruiken gel-polymeer-elektrolyt voor hoogwaardige magnesiumbatterijen
Wetenschappers gebruiken gel-polymeer-elektrolyt voor hoogwaardige magnesiumbatterijen Begrijpen hoe de monomeersequentie de geleiding in 'moleculaire draden' beïnvloedt
Begrijpen hoe de monomeersequentie de geleiding in 'moleculaire draden' beïnvloedt
Hoofdlijnen
- Hoe het influenzavirus efficiënte virale RNA-replicatie bereikt
- Wat is een unitellulair organinsman?
- Beschrijf hoe wordt de structuur van de eiwitten bepaald door de opstelling van aminozuren?
- Van vis tot mens:onderzoek laat zien hoe vinnen benen werden
- Op welke toestand moeten dieren die in zoetwaterecosystemen leven zich aanpassen?
- Herziening van historische voorraadroutes kan zeldzame stukken inheemse planten en dieren in gevaar brengen
- Van welke waarde is een capsule voor bacterie?
- Wat is de definitie van lysosoom?
- Wat is de koloniemorfologie van Edwardsiella Tarda?
- Toekomstige informatietechnologieën:3D-kwantumspinvloeistof onthuld

- Neutrino-ontdekking - een stap dichter bij het vinden van een overtreding van de ladingpariteit

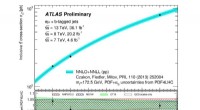
- Inzoomen op de productie van top-quark
- Een nieuwe theorie van supergeleiding

- Nieuw optisch apparaat kan helpen bij het opsporen van drugs, chemicaliën voor het maken van bommen en meer

 Hoe stormjagers werken
Hoe stormjagers werken  Bestaat er een fenomeen dat het golfkarakter van licht niet ondersteunt?
Bestaat er een fenomeen dat het golfkarakter van licht niet ondersteunt?  Hoe zou u metalloïde of niet-metaal van zwavels-metaal classificeren?
Hoe zou u metalloïde of niet-metaal van zwavels-metaal classificeren?  Wat beïnvloedt de sterkte van een elektrisch veld?
Wat beïnvloedt de sterkte van een elektrisch veld?  Welk type verwering zorgt ervoor dat de minerale samenstellingsrotsen veranderen?
Welk type verwering zorgt ervoor dat de minerale samenstellingsrotsen veranderen?  Studie vindt delta helpt de impact van rivieroverstromingen te verminderen
Studie vindt delta helpt de impact van rivieroverstromingen te verminderen Wat is het menselijke voortplantingssysteem gereguleerd door?
Wat is het menselijke voortplantingssysteem gereguleerd door?  Welke twee luchtmassa's zijn in de winter de VS?
Welke twee luchtmassa's zijn in de winter de VS?
- Elektronica
- Biologie
- Zonsverduistering
- Wiskunde
- French | Italian | Spanish | Portuguese | Swedish | German | Dutch | Danish | Norway |

-
Wetenschap © https://nl.scienceaq.com