Wetenschap
Why can water dissolve so many compounds?
1. Polarity: Watermoleculen zijn polair, wat betekent dat ze een enigszins positieve lading hebben aan het ene uiteinde (waterstofatomen) en een enigszins negatieve lading aan de andere kant (zuurstofatoom). This polarity allows water to interact with and dissolve other polar molecules, such as sugars and salts.
2. Hydrogen Bonding: De enigszins positieve waterstofatomen in één watermolecuul kunnen zwakke bindingen vormen die waterstofbruggen worden genoemd met het enigszins negatieve zuurstofatoom van een ander watermolecuul. This strong network of hydrogen bonds gives water its high cohesive forces and surface tension, which helps it pull apart ionic compounds.
3. High Dielectric Constant: Water has a high dielectric constant, meaning it can effectively weaken the electrostatic forces holding ions together in ionic compounds. This allows water to separate the ions and surround them individually, effectively dissolving the compound.
4. Universal Solvent: Water's ability to dissolve a wide range of substances makes it a "universal solvent". This property is crucial for life, allowing for the transport and reaction of nutrients and waste products in living organisms.
Specifieke voorbeelden:
* Ionic Compounds: Water kan ionische verbindingen zoals natriumchloride (NaCl) oplossen door de individuele ionen (Na+ en Cl-) te omringen en hun elektrostatische aantrekkingskracht te verzwakken.
* Polar Molecules: Water can dissolve polar molecules like sugar by forming hydrogen bonds with the polar groups on the sugar molecule.
* Gases: Some gases, like carbon dioxide, can dissolve in water due to their ability to form weak interactions with water molecules.
Limitations:
While water is an excellent solvent, it doesn't dissolve everything. It has difficulty dissolving nonpolar substances like fats and oils due to their lack of polar groups that water can interact with.
Concluderend, de unieke combinatie van water van polariteit, waterstofbinding, hoge diëlektrische constante en zijn vermogen om verschillende interacties met verschillende moleculen te vormen, maakt het een krachtig en veelzijdig oplosmiddel. This property is essential for many biological and chemical processes, making water an indispensable component of life on Earth.
 Een krabbenperspectief van opkomend tij in een veranderende wereld
Een krabbenperspectief van opkomend tij in een veranderende wereld Nieuwe toolvondsten en vingerafdrukken die voorheen onopgemerkte PFAS-verbindingen waren in stroomgebieden op Cape Cod
Nieuwe toolvondsten en vingerafdrukken die voorheen onopgemerkte PFAS-verbindingen waren in stroomgebieden op Cape Cod Waar wordt de koolstof van de aarde opgeslagen?
Waar wordt de koolstof van de aarde opgeslagen?  Onderzoek kan verklaren waarom wolven voor altijd wild zijn, maar honden kunnen getemd worden
Onderzoek kan verklaren waarom wolven voor altijd wild zijn, maar honden kunnen getemd worden  Ontsnap aan de dampen:wetenschappers roepen op tot een mondiale verschuiving om het consumentengebruik van wegwerptechnologieën te beteugelen
Ontsnap aan de dampen:wetenschappers roepen op tot een mondiale verschuiving om het consumentengebruik van wegwerptechnologieën te beteugelen
Hoofdlijnen
- What are the secretory cells of an ovarian follicle called?
- Wat wordt biologische evolutie gebruikt om uit te leggen?
- Nieuwe studie ontdekt hoe veranderde eiwitvouwing de meercellige evolutie aandrijft
- Welk type metabolisme doet Archaea?
- Waarom zijn het allemaal levende wezens afkomstig van een gewone of universele voorouder?
- Wat is een organisme dat niet op zichzelf kan leven?
- Wat is de term voor een paddestoelachtige groei die zich uitstrekt op een stengel van het oppervlak van het slijmmembraan?
- Welke verbinding die is afgeleid van glucose komt daadwerkelijk de Krebs -cyclus binnen?
- Hoe beïnvloedt het endocriene systeem het uitscheidingssysteem?
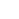
- Niet-invasieve magnetische resonantiebeeldvorming van longfibrogenese met een op aminozuren gerichte sonde
- Nieuwe polymeermembraantechnologie verbetert de efficiëntie van het afvangen van kooldioxide

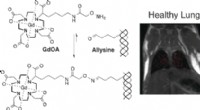
- Cholesterol helpt het griepvirus te ontsnappen door het membraan van de gastheercel
- Detectie van kanker door uitgeademde adem

- Hittegeharde magnesiumlegering een sterke keuze voor implantaten
 Wat is de rol van moleculaire werking bij convectie en geleiding?
Wat is de rol van moleculaire werking bij convectie en geleiding?  Waarom zijn sommige sterren groter dan andere?
Waarom zijn sommige sterren groter dan andere?  Studie onderzoekt op welke zoekopdrachten autokopers klikken
Studie onderzoekt op welke zoekopdrachten autokopers klikken Waarom komen aardbevingen op langs fouten?
Waarom komen aardbevingen op langs fouten?  Storm laat 3 gewonden achter, duizenden ontheemden in de Filippijnen
Storm laat 3 gewonden achter, duizenden ontheemden in de Filippijnen Hoe een vloeistof kan worden gewijzigd in gasvorming van onder het boorpunt zonder deze te voorzien van warmte -energie?
Hoe een vloeistof kan worden gewijzigd in gasvorming van onder het boorpunt zonder deze te voorzien van warmte -energie?  Wat zijn 3 manieren waarop een berg kan worden gevormd?
Wat zijn 3 manieren waarop een berg kan worden gevormd?  Elektrische auto's zijn een gevaar voor blinden
Elektrische auto's zijn een gevaar voor blinden
- Elektronica
- Biologie
- Zonsverduistering
- Wiskunde
- French | Italian | Spanish | Portuguese | Swedish | German | Dutch | Danish | Norway |

-
Wetenschap © https://nl.scienceaq.com