Wetenschap
Would two compounds made of nitrogen and oxygen always be identical?
* Different Ratios: Nitrogen and oxygen can combine in different ratios to form different compounds. Bijvoorbeeld:
* Nitrous oxide (N₂O) has two nitrogen atoms and one oxygen atom.
* Nitrogen dioxide (NO₂) has one nitrogen atom and two oxygen atoms.
* Different Structures: Zelfs als de verhouding van stikstof en zuurstof hetzelfde is, kan de opstelling van atomen (de moleculaire structuur) verschillen, wat leidt tot verschillende eigenschappen.
* Isomers: There are cases where two molecules have the same chemical formula but different arrangements of atoms. These are called isomers, and they can have different physical and chemical properties.
Therefore, the identity of a nitrogen and oxygen compound depends on both the ratio of elements and the molecular structure.
 Kun je waterstof en zuurstof creëren zonder water te gebruiken?
Kun je waterstof en zuurstof creëren zonder water te gebruiken?  Chemische reacties voltooien
Chemische reacties voltooien Oxiden reageren met zuurstof en water worden gebruikt om verf enkele eigenschappen te maken welk type metalen?
Oxiden reageren met zuurstof en water worden gebruikt om verf enkele eigenschappen te maken welk type metalen?  Wat is het verschil in structuur tussen een verzadigde en een onverzadigde verbinding?
Wat is het verschil in structuur tussen een verzadigde en een onverzadigde verbinding?  Wat is de uitgebalanceerde vergelijking voor de verbranding van magnesium?
Wat is de uitgebalanceerde vergelijking voor de verbranding van magnesium?
 Alle levende en niet -levende elementen op een bepaalde plaats worden een (n) genoemd?
Alle levende en niet -levende elementen op een bepaalde plaats worden een (n) genoemd?  Waarom zwemmen zalm en andere vissen stroomopwaarts?
Waarom zwemmen zalm en andere vissen stroomopwaarts?  Wat zijn twee dingen in de natuur dat periodiek?
Wat zijn twee dingen in de natuur dat periodiek?  Wat zijn dam-overstromingsgebieden?
Wat zijn dam-overstromingsgebieden?  Onderzoekers ontdekken dat ijs naar de randen van de Groenlandse ijskap glijdt
Onderzoekers ontdekken dat ijs naar de randen van de Groenlandse ijskap glijdt
Hoofdlijnen
- Is een intense concurrentie om een voedselbrondichtheid afhankelijk of onafhankelijk?
- Het stellen van minimumdoelen voor natuurbehoud sluit herstel en ecosysteembeheer uit, betoogt de onderzoeker
- Als een bacterie geen glucose kan fermenteren, waarom dan zijn vermogen niet testen op andere koolhydraten?
- Wat is de rol van EtBr bij elektroforese?
- Op welke twee manieren kunnen delen van de biosfeer interageren met de hydrosfeer?
- Een 3D-poster maken van de celcyclus
- Eukaryotische cel: definitie, structuur en functie (met analogie en diagram)
- Lasertechnologie biedt doorbraak in het opsporen van illegaal ivoor
- DNA in Vikingpoep werpt nieuw licht op 55.000 jaar oude relatie tussen darmgenoten
- Ingenieurs vinden dat flessenborstelcopolymeren kunnen worden aangepast voor toepassingen

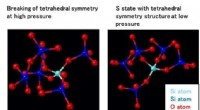
- Structurele oorsprong van de afwijkende eigenschappen van SiO2-glas onder druk
- Naar een door licht aangedreven moleculaire assembler

- Wetenschappers melden voor het eerst zware ionenoverdracht in geladen vdW-cluster

- Onderzoekers bestuderen historische ontwikkelingen van het periodiek systeem van chemische elementen

 Welke levensprocessen worden door planten en dieren gebruikt om energie te verkrijgen?
Welke levensprocessen worden door planten en dieren gebruikt om energie te verkrijgen?  Groot aantal volwassenen dat niet in staat is om elementaire wiskundige taken uit te voeren
Groot aantal volwassenen dat niet in staat is om elementaire wiskundige taken uit te voeren Boventonen kunnen zorgen voor snellere datacommunicatie
Boventonen kunnen zorgen voor snellere datacommunicatie Fox zegt dat Disney Sky News kan kopen in een nieuwe overnamedraai
Fox zegt dat Disney Sky News kan kopen in een nieuwe overnamedraai Wat zijn de beperkingen van Covalent & Metallic Roosters?
Wat zijn de beperkingen van Covalent & Metallic Roosters?  Hoeveel schillen hebben 20 elektronen nodig?
Hoeveel schillen hebben 20 elektronen nodig?  Jack the Ripper en de commercialisering van seksueel geweld
Jack the Ripper en de commercialisering van seksueel geweld Onderzoekers bestuderen de ondoorzichtige accretieschijf van Beta Lyrae A
Onderzoekers bestuderen de ondoorzichtige accretieschijf van Beta Lyrae A
- Elektronica
- Biologie
- Zonsverduistering
- Wiskunde
- French | Italian | Spanish | Portuguese | Swedish | German | Dutch | Danish | Norway |

-
Wetenschap © https://nl.scienceaq.com